Biosecurity
Biosecurity
A comprehensive biosecurity program based on thorough verification of the biological safety of an object or process together with a risk with mitigation plan.
BioPlan is always based on on-site visit, and comprehensive analysis of the epizootic situation together with the assessment of biosecurity safeguards.
bioplan
on-site audit
On-site audit: a visit to the facilities throughout the world, duration about 5 hours depending on the size and complexity of the facility. Guaranteed downtime for contact with pigs – 12h. Detailed conditions to be agreed.
Assessement
Biosecurity system assessment based on the Biocheck software developed by the University of Ghent, the questionnaire contains over 100 questions, allows the assessment of internal and external biosecurity. Assessment confirmed by a written report / certificate.

Analysis
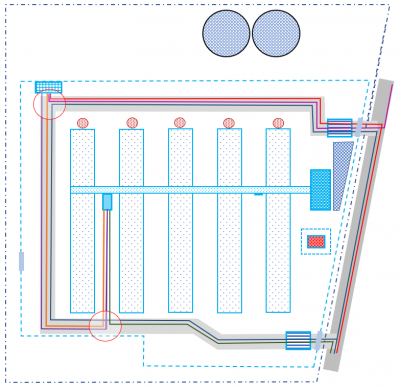
Biological safety level analysis – based on the Biocheck assessment as well as on the epizootic situation of the facility and geographical location. The dependencies and influences to which a given object is subject are also analysed. The analysis includes preparation of the biosecurity map of the object.
Mitigation Plan
Mitigation plan – it is developed respecting the guidelines supported by scientific research and the latest know-how. It is very important here to respond to the specific needs, capabilities and location of the object, and to act with strictly defined priorities. Recommendations are always prepared individually for each facility after the analysis of biological hazards and customer needs.
biosecurity program
A comprehensive biosecurity program and production procedures – this is an individually developed comprehensive biosecurity program for one facility or vertically integrated systems with a set of operational procedures. The developed procedures cover the entire scope of external and internal biosecurity.
support
Support in the implementation of BioPlan and training for employees – available as an option, for individual development, possible onsite and online.
audit of entities
Audit of subsidiaries and suppliers – available as an option, to be individually developed depending on the risk analysis.
After completing the entire BioPlan cycle, it is recommended to conduct a BioPlan compliance audit once a year in order to maintain the developed standard and crew mobilization.
Financial and insurance institutions:
BioPlan can be used as a confirmation of the level of biosecurity, and thus be a part of the calculation of an insurance sum, or it can be used as an part of an investment project.

BioPlan is dedicated to:
• individual farms
• integrated producers
• breeding companies
• feed mills
• slaughterhouses
• washing facilities
• transport companies
• AI stations
• approved animal rest points
• meat producers
• financial and insurance institutions
• companies supporting animal production
BIOMAP